
In the case of Blincyto, the drug attaches itself to a protein called CD19, which exists on B cells. These drugs connect proteins in T cells (the immune cells that kill ca ncer and infected cells) to proteins in B cells (the malignant cells in B-cell ALL). How Does BiTE Treatment Work?īiTE treatment works by inducing cytotoxic T cells to fight tumor cells. Even if a person seems clinically to be in remission, persistent MRD is the strongest predictor of an eventual poor prognosis. These new tests are powerful enough to detect 1 cancer cell among 1 million normal cells. However, recently developed molecular assay testing allows the detection of MRD - tumor cells that would escape conventional microscope testing. Hematological remission means that the bone marrow contains an appropriate amount of white blood cells and that there are no signs or symptoms of leukemia. It is also approved for people who are in hematological remission from B-cell ALL, but still show minimal residual disease (MRD). Food and Drug Administration (FDA) for people with refractory or relapsed B-cell acute lymphoblastic leukemia (ALL). Blincyto (blinatumomab) is approved by the U.S. Bispecific T-Cell Engager (BiTE) TreatmentĬurrently, only one form of BiTE treatment is available to treat leukemia. The following sections describe BiTE treatment and CAR-T cell therapy in greater detail.
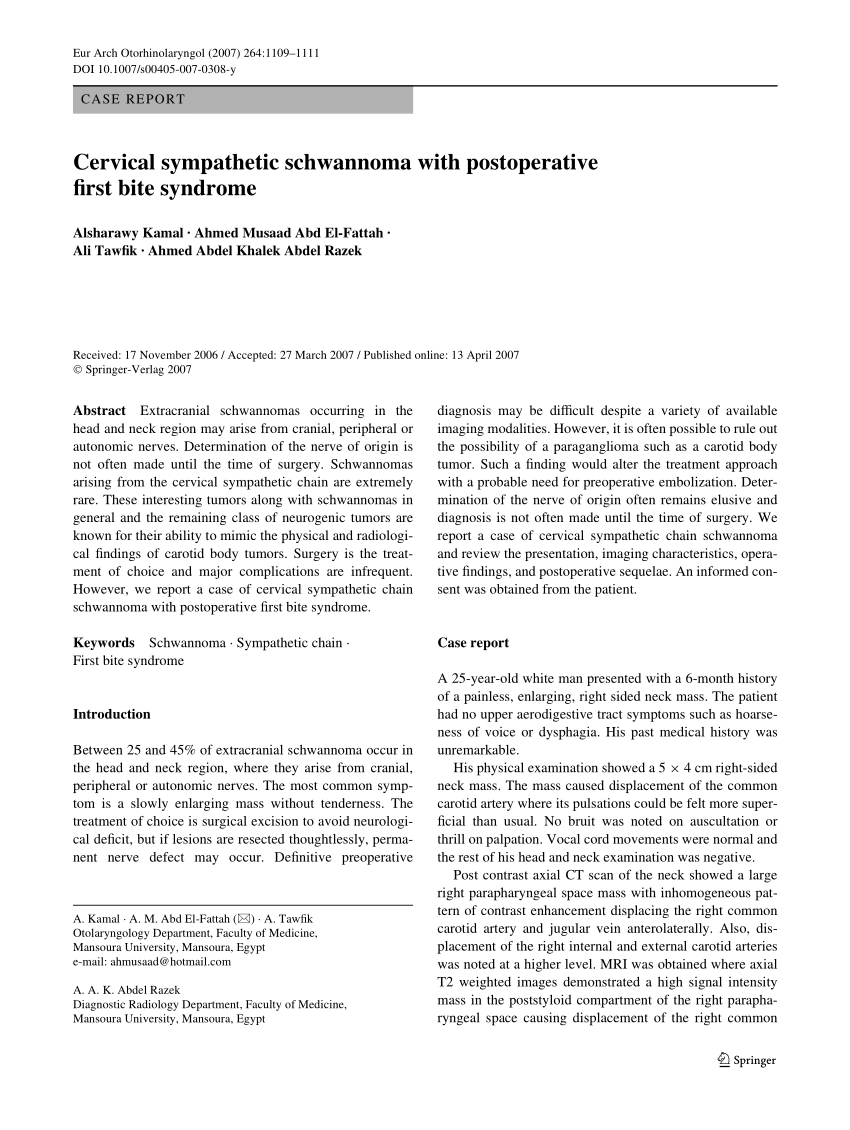
Patients undergoing surgery with dissection and/or manipulation in these anatomical sites and structures should be thoroughly counseled about the risk of developing FBS.Ĭopyright © 2012 The American Laryngological, Rhinological, and Otological Society, Inc.This chart compares the major differences and similarities between BiTE treatment and CAR-T cell therapy treatment for leukemia. The strongest independent risk factors for FBS are PPS dissection, deep lobe of parotid resection, and sympathetic chain sacrifice. No treatment consistently provided effective symptomatic relief. Of 45 FBS patients, 15 (33%) underwent at least one type of treatment for symptomatic relief. Partial resolution of FBS symptoms occurred in 69% and complete resolution in 12%. FBS developed in 48.6% of patients undergoing sympathetic chain sacrifice, 22.4% of patients undergoing PPS dissection, 38.4% of patients undergoing isolated deep lobe parotid resection, and 0.8% of patients undergoing total parotidectomy. 001), and resection of only the deep lobe of the parotid gland (OR, 4.2 P =. On multivariate analysis, three variables were significant independent risk factors for FBS: sympathetic chain sacrifice (odds ratio, 4.7 P =. Patients developing FBS were interviewed to assess the efficacy of various treatment modalities.įBS developed in 45 patients (incidence, 9.6%), at a mean time of 97 (range, 6-877) days from surgery.
Univariate analyses and multivariate logistic regression were used to identify independent risk factors for FBS. Incidence was calculated using the Kaplan-Meier method.
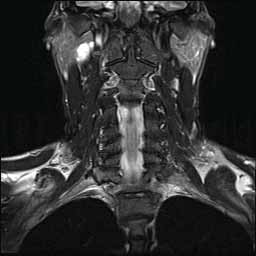
Patient, tumor, and FBS characteristics were analyzed. Minimum follow-up time was 3 months (median, 39 months). We reviewed the records of 499 patients (mean age, 50 years range, 12-81 years) undergoing surgery of the deep lobe of the parotid gland, PPS, and/or ITF between 19. We hypothesized that certain clinical and tumor variables independently predict the development of FBS. The incidence, risk factors, treatment options, and outcomes of FBS are poorly understood. First bite syndrome (FBS) refers to facial pain characterized by a severe cramping or spasm in the parotid region with the first bite of each meal that diminishes over the next several bites.1, 2 It is a potential sequela of surgery involving the infratemporal fossa (ITF), parapharyngeal space (PPS), and/or deep lobe of the parotid gland.


 0 kommentar(er)
0 kommentar(er)
